Posted by: Christa Wagner, 2016-17 ASHG/NHGRI Genetics & Public Policy Fellow
Starting this month, the grant application process for NIH-funded research that includes human subjects will change for many investigators. This stems from modifications NIH has made to its definition of a clinical trial, and a number of new requirements the agency is establishing for investigators conducting NIH-funded clinical trials. It is important that researchers understand these changes and consider whether their research is now regarded as a clinical trial by NIH.
What is Defined as a Clinical Trial?
NIH’s new definition went into effect in January 2015 and states that a clinical trial is:
“A research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.”
Many scientists think of clinical trials as investigations of the safety and effectiveness of potential clinical interventions. For instance, the National Heart, Lung, and Blood Institute has defined clinical trials as “research studies that explore whether a medical strategy, treatment, or device is safe and effective for humans”. In contrast, the new NIH definition includes research projects that don’t take place in a clinical setting and those that do not test a new treatment – studies that some researchers regard as basic research. This is best demonstrated by case studies the NIH has issued to illustrate what types of research fall within its new definition.
New Requirements for Researchers Conducting Clinical Trials
Understanding whether NIH defines your research as a clinical trial is important because the agency is setting new requirements for clinical trial investigators. These changes affect the grant application process and grant review, as well as the awarding of funding; training of staff conducting clinical trials; management and oversight of a funded trial; public registration of the trial; and timely dissemination of results. Below is a list of relevant policy changes, their effective date, and how they could impact your research and funding.
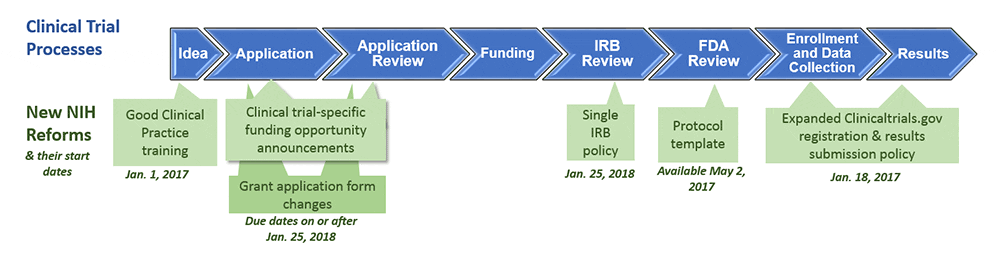
- Definition of clinical trial – January 1, 2015. The new definition (see above) expands the scope of what is considered a clinical trial, encompassing more research than the previous definition. For example, it may include many ELSI research projects, such as feasibility studies and studies comparing consent approaches. Read this blog post from the NIH, this decision guide, or the list of case studies if you are unsure of how to categorize any research you conduct with human subjects. Of particular interest to the ASHG community are case studies 4, 7, 10, 11, 13, and 20.
- Good Clinical Practice (GCP) training – January 1, 2017. This policy requires PIs and staff involved in NIH-funded clinical trials to receive training in good clinical practice as a condition of the award.
- Clinical trial protocol template – May 2, 2017. NIH and FDA collaborated on a new template for Phase 2 and 3 Investigational New Drug (IND)/Investigational Device Exemption (IDE) clinical trials.
- Registering clinical trials and reporting results – January 18, 2017. Any clinical trial receiving NIH funding must register on ClinicalTrials.gov within 21 days of enrolling its first participant. Furthermore, results from a clinical trial must be submitted to the same website within one year of the trial’s completion. Failure to do so may result in the NIH withholding grant funding from the grantee institution. Read more about registering and reporting of research.
- Applying for NIH funding for clinical trials research – January 25, 2018. This month, all Funding Opportunity Announcements (FOAs) will be changed to accommodate the new definition of clinical trial. For grant applications due on or after January 25, 2018, research projects now deemed a clinical trial must be submitted to a new category of FOA specifically designated for clinical trials research.
- Use of single Institutional Review Board (sIRB) – January 25, 2018. This policy applies to multi-site research proposals.
We encourage you to look into how these changes will affect your NIH-funded research with human subjects. Please feel free to let us know your questions, comments, or concerns by posting below or emailing policy@ashg.org.
Additional Information from NIH Blogs and Publications
- September 2016 statement and rationale for clinical trials updates
- October 2016 JAMA publication from NIH authors discussing rationale and description of the changes to clinical trials research definitions and procedures
- October 2016 blog post on implications of the new clinical trials definition for behavioral and social science researchers
- January 2017 update on the NIH clinical trials initiative
- August 2017 update on defining clinical trials research
- September 2017 updates for clinical trials researchers
