Posted By: Christa Wagner, PhD, 2016-17 ASHG/NHGRI Genetics & Public Policy Fellow
With rapid DNA sequencing now routine, genetics researchers are increasingly testing the clinical utility of its application in various healthcare settings and the barriers to using genomic information in healthcare decision-making. In doing so, some are discovering that their research might be subject to a Food and Drug Administration (FDA) regulation known as the investigational device exemption (IDE) regulation. However, many in the genetics community are unfamiliar with this regulation.
To raise awareness of the IDE regulation, ASHG hosted a policy luncheon at ASHG 2017 that included representatives from the FDA, the research community, and the National Human Genome Research Institute (NHGRI). Presenters explained the relevance of the regulation for genomics research, described an experience of applying for an IDE, and made attendees aware of helpful resources available to the genomics community.

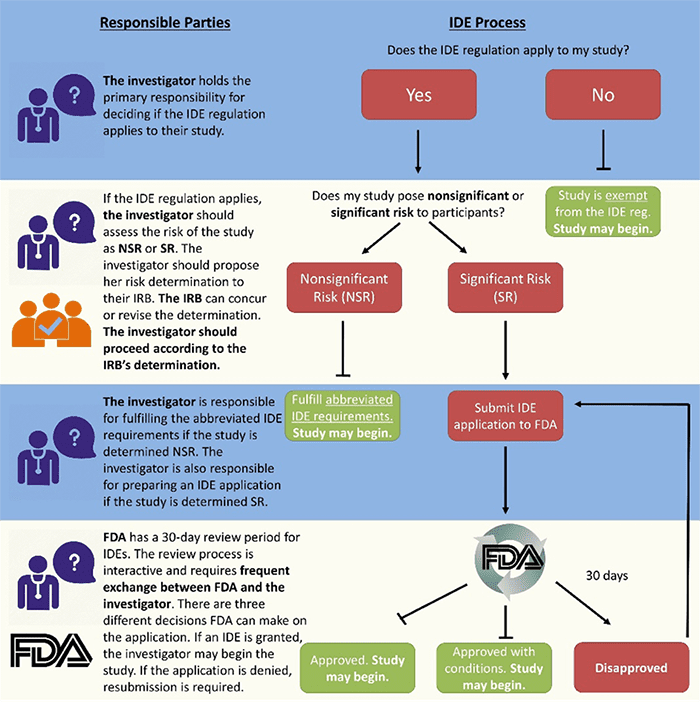
Katherine Donigan, PhD, from the Personalized Medicine Staff at FDA began the session by speaking about the IDE regulation itself and her office’s role in ensuring public health and safety while supporting innovative research and discovery. She explained that genomics research has made great advancements since the IDE regulation went into effect in 1976, and that research using next-generation sequencing where results are being returned to participants warrants consideration under the IDE regulation.
Studies can fall into one of three categories: IDE exempt, Nonsignificant Risk (NSR), and Significant Risk (SR). Only SR studies require FDA involvement, and Kate outlined the variables that her team uses to determine SR, such as the invasiveness of sample collection and the potential for false results. She advised researchers to reach out to FDA to talk through their proposed studies before applying for a grant, so that they know in advance if their research will likely be categorized as SR. This will facilitate a faster IDE application and approval process once grant funding has been awarded.
Jonathan Berg, MD, PhD, from the University of North Carolina then presented on his experience as a trailblazer in interacting with FDA regarding IDE regulations for genomic studies. Jonathan’s NC NEXUS project in the NSIGHT consortium explores the utility of exome sequencing for enhancing newborn screening by sequencing both healthy newborns and infants previously identified through newborn screening, and studying parental decision-making on the additional genomic testing. He detailed the multi-year long process his research group struggled through to become aware of, apply for, and eventually receive an IDE approval. Jonathan explained that he is supportive of FDA oversight but that the regulations need to be applied in a way that does not derail scientific research. He offered some words to the wise: get help! Discuss the risks involved in your research studies with your IRB or other institutional regulatory assistance staff, or consult with the FDA.
Cristina Kapustij, MS, from NHGRI rounded out the panel by outlining the support role her policy team offers to stakeholders and grantees embarking on human genetics research. She explained that NHGRI is working as a bridge to the FDA for researchers looking for guidance on the IDE process when they are designing studies or applying for grants. She described a workshop hosted by NHGRI in 2016 and drew attention to the NHGRI Points to Consider online resource.
The panel concluded with a Q&A session. Attendees asked great questions about diverse topics from funding to return results, retrospective research with biobank samples, streamlining the IDE submission process as technology continues to advance, and more! Check out the information below for more details on IDEs.
Resources
ASHG 2017 Policy Luncheon
- Kate Donigan, FDA, presentation video | slides
- Jonathan Berg, UNC, presentation video | slides
- Cristina Kapustij, NHGRI, presentation video | slides
- Panel discussion moderated by Derek Scholes, ASHG Director of Science Policy
NHGRI
- Points to Consider online resource regarding IDE regulations in the context of genomics research
- Proceedings of 2016 NHGRI workshop on IDEs and genomics
- Federal regulation of genetic tests
FDA
- Device Advice on the IDE regulation
- CDRH Learn resource with multiple presentations on IDEs
Christa Wagner, PhD, is the 2016-17 ASHG/NHGRI Genetics & Public Policy Fellow. For more information on the fellowship and application details, please visit the Genetics & Public Policy Fellowship website.
