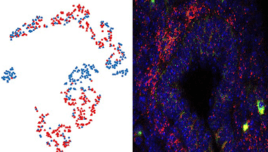
Biology at a Single Cell Resolution: An Idea Whose Time Has Come
Written by Nascent Transcript writer Sumantra Chatterjee, PhD February 2018 Almost 180 years ago, Theodor Schwann, Matthias Schleiden, and Rudolf Virchow laid down three fundamental tenets of biology collectively called the “cell theory”. Since then, the principles that the cell is the most basic unit of life, all living organisms are composed of cells, and... Read More